An optimally functioning digestive system is INDISPENSABLE when it comes to maintaining balanced pH levels and overall health. A healthy digestive system breaks down food and makes nutrients available to the body for growth, healing, and everyday functioning, while eliminating waste. When this life-giving system is distressed, EVERY other body system is affected in some way, making pH balance and overall wellness impossible!
There are many key factors that need to be addressed for this system to be able to perform satisfactorily including: pH balanced anti-inflammatory diet, sufficient gastric juices, balanced intestinal pH levels, physical movement, stress management, limiting environmental toxic exposure, and regular cleansing. All of these things can be achieved through self-education, discipline, and conscious living.
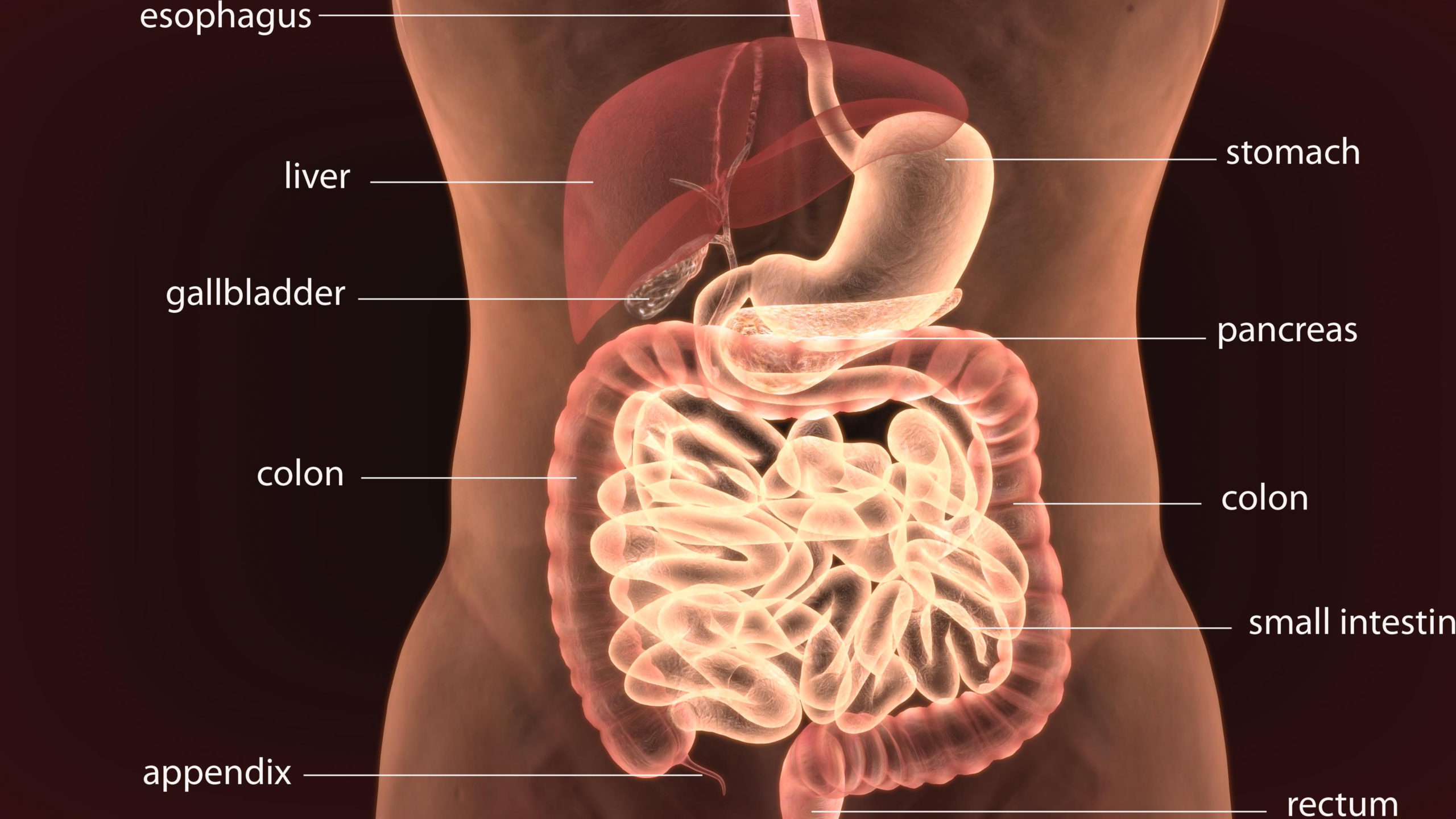
Digestion entails a long and intricate process. Simply put, digestion begins in the mouth and ends in the colon. pH levels vary at different phases throughout the digestive process. When in balance, the mouth’s pH ranges from about 5.5 – 7. It is slightly on the acidic side so that amylase, the enzyme responsible for initiating the breakdown of starch, can be activated. Moving along, whatever has been consumed passes down the esophagus and waits in the upper stomach, while the lower stomach (pH ranges from 1.5 – 4), prepares for the next phase of digestion by secreting gastric juices like hydrochloric acid (HCL).
Almost as powerful as battery acid, HCL acts as the first line of defense, protecting you from bacteria and other pathogens that may be present in the food or beverages ingested. This potent acid breaks down the stomach’s contents into a thick liquid known as chime, preparing for the next phase of digestion. After adequate HCL is secreted, pepsin enzymes are released to break down proteins that will be converted into useable compounds for the body. Then alkaline bicarbonates are released from the pancreas. These bicarbonates help prepare the chime for its entry into the slightly acidic small intestine via the duodenum (opening to the small intestine).
Digestion then continues into the intestines, starting with the small intestine. The pH of the small intestine fluctuates from 6 – 7 or so to breakdown nutrients further and to better facilitate absorption. As this now higher pH mixture enters the small intestine, the pancreas is signaled to release digestive enzymes to complete the digestion and assimilation of 90 – 95% of the nutrients. When in balance, alkaline bile is then secreted by the gallbladder/ liver to help breakdown fats and rid the body of various waste products. To finish off the process, the remaining water and nutrients are absorbed through a slightly acidic colon in the large intestine, with a pH ranging from 5.5 – 6.7. Finally, the waste material that is left over is eliminated through the rectum, and the excess water and electrolytes are absorbed.
When pH levels remain in normal range throughout each phase of the digestive process, the result is optimal absorption and assimilation of ingested foods and supplements.
Additionally, this complex intestinal system houses a significantly large percentage of your immune health by maintaining a symbiotic balance of microbiome. These gut bacteria directly influence genetic activity and interact intimately with the central nervous system, referred to as the gut-brain connection. We will dive much deeper into this subject matter in (the relevance of gut bacteria section). With that being said, balanced pH levels throughout the digestive system enhance and strengthen the immune system and directly impact our memory, mood, appetite, behavior, emotions, hormones, stress response, and the entire genome!
THE DIGESTIVE SYSTEM’S ASSISTANT ORGANS & THEIR NEED FOR BALANCE
The digestive system does not work alone but has three additional organs that assist in the digestive process. They are the liver, gallbladder and pancreas. These organs are anatomically positioned right near each other, working together to assist the digestive process. They (like all other systems in the human body), must remain within their ideal pH range in order to operate optimally. The pancreas and the liver should be slightly alkaline, with the pH of pancreatic juice being around 8.0, and the bile from the liver holding between 7.5 and 8.8.
The liver is involved in the production of this substance called bile, which is used to buffer stomach content, process fats, and eliminate toxic acidic waste. The bile is stored in the gallbladder, with the majority of its release following a fat containing meal. When you consume fats, the bile is then released into the small intestine, assisting in the breakdown of these fats to then be utilized by the body.
HCL helps to break down food into chime and release pepsin to start dismantling proteins. Then the pancreas is signaled to secrete or release bicarbonates to neutralize the acidic chime for its entry into a more alkaline small intestine along with the bile. Once there, enzymes trypsin and chymotrypsin are released to assist protein digestion, amylase for carbs, and lipase for fats. The pancreas is also responsible for releasing insulin and glucagon to help regulate the amount of sugar in the blood and body cells.
As you have learned, the digestive system’s assistant organs have specific pH levels that must hold within a certain range for optimum functioning. High acid levels have direct detrimental effects. The following are some of the consequences that acid/alkaline imbalances have on these organs:
- When the acidity of the blood and body tissues increases, the acidity of pancreatic juices and bile do as well. When this happens, the tissues of the liver and pancreas can become inflamed and their function compromised, potentially forming ulcers, creating the perfect environment for cancer.
- When the bile becomes overly acidic, bile reflux can result. This causes the bile to back up through the pancreatic duct, followed by the small intestine, and then up into the stomach and esophagus. This can cause severe inflammation with the potential for ulcers and cancer to form. A lot of times acid reflux and bile reflux happen simultaneously, specifically increasing the risk of esophageal cancer.
- When foods are allowed to ferment in the gut, undigested rotting foods and toxic bi-products make their way to the liver creating acidic bile. This prevents the duodenum (the entrance to the small intestine), from releasing alkaline chime for further breakdown and absorption in the small intestine.
- Acidic bile is also a major contributor in the development of gallbladder sludge and stones. The stones can then block pancreatic and bile ducts causing immense pain as well as damage to these organs. Gallstones can prevent the liver from producing enough bile, leading to malnutrition and mineral deficiencies.
- Without enough alkaline bile being secreted, it is very hard for the intestines to maintain specific pH levels. This, in turn, causes inflammation of the intestinal walls, potentially leaking undigested foods, toxins, and acidic waste into the bloodstream and wreaking havoc on all body systems.
- When the blood and body tissues are too acidic, and the pancreatic juices aren’t as alkaline as they should be, fewer enzymes are produced. Lack of enzymes causes indigestion, an increased risk of calcium stones blocking the pancreatic duct, and in the worst cases, leads to pancreatitis. With pancreatitis, enzymes become active prematurely inside the pancreas, which actually causes the pancreas to digest its own cells!
- Enzymes are a part of just about every biochemical process in the body. They are often so target-specific and complex that they are likened to “keys” that only fit certain key holes to be able to carry out their specific function. If tissue and fluid pH is even slightly off balance, the keys will not be able to open the door efficiently. If the target specific environmental pH is too acidic (low pH) or alkaline (high pH) enzymes won’t function or will become denatured. The skin, GI tract (especially the stomach) and vaginal area require a lower pH, and the rest of the body like the blood, interstitial fluids, and other organ systems, need to be on the alkaline side. Too high or too low intestinal pH stunts enzyme activity and leads to poor absorption and assimilation of nutrients. Also, pepsin (the enzyme that digests protein in the stomach), requires a pH around 2.
For example, enzymes in the skin are responsible for its thickness. The optimal pH of the skin is around 5.5 to help thin out older skin cells so new ones can grow. The enzymes are pH sensitive, so if the skin pH rises only 2 points from 5.5 to 7.5, the increase of the thinning enzyme activity is 50%! Besides aging skin, now you’re more susceptible to allergens and pathogenic bacteria, which can lead to skin conditions like eczema. When enzymes are off, body systems overall will suffer, period.
